This is excerpted from the following document:
Durban Declaration Rebuttal
A
rebuttal to the "Durban Declaration"
published in Nature on July 6 2000.
Revised September 28, 2001
Compiled by Robert Johnston1, Matthew Irwin2
and David Crowe3
1: Co-founder of
HEAL Toronto, 2: Co-founder of HEAL
Washington DC, 3: President of the Alberta Reappraising AIDS Society.
THE RELIABILITY
OF HIV ANTIBODY TESTS
We are often reminded by HIV researchers that HIV
has a relationship to AIDS. Certainly there is a loose correlation between HIV-positive
antibody results and AIDS, but it is not enough to fulfill Koch's first postulate.
Perhaps the reason the HIV antibody tests tend to identify AIDS patients is
that it functions like the erythrocyte sedimentation rate (ESR) test and other
non-specific antibody tests like rheumatoid factor (RF), and anti-nuclear antibody
(ANA) tests (Turner, 1997). After all, no one has ever established a gold standard,
such as purification of HIV from fresh patient plasma or even cell culture,
as Luc Montaignier admits (Tahi,
1997). Therefore, any of 70 conditions already identified could be the cause
of "HIV positivity" in any or all cases
(Johnson, 1998). The erythrocyte sedimentation rate (ESR) is a blood test that
is frequently ordered in internal-medicine and infectious-disease offices. The
test measures the rate that red blood cells (erythrocytes) fall to the bottom
of a test tube under controlled conditions. An increased rate, usually defined
as greater than 10 in a young adult, generally reflects an inflammatory process
somewhere in the body. The ESR can be elevated in a multitude of disorders,
from a mild infection, to a severe infection, malignancy or even kidney failure.
A normal ESR can virtually exclude some disorders, but it is never diagnostic
of any one particular disease.
Other very non-specific antibody tests like the Rheumatoid Factor (RF) and the
Anti-nuclear Antibody (ANA) tests, are positive mostly
in people with certain autoimmune diseases, but are also often positive in healthy
adults. These tests use diluted serum, because otherwise most people would test
positive. They are not as nearly diluted as the 400-fold dilution of the HIV
ELISA test, however. When the ELISA is run on straight serum, as is done for
the majority of antibody tests like hepatitis B antibodies, 100 out of 100 HIV-negative
blood samples became positive! (Giraldo, 1998/1999)
The Western Blot also uses diluted serum, but no studies could be found by these
authors in which it was tested on straight serum. This would be an essential
test of the Western blot's specificity, especially given that even when diluted,
indeterminate readings are quite common. An article in a leading journal presents
this issue:
Problems may be
encountered when an HIV Western Blot is done on someone at no identifiable risk
of infection. For example, recent studies of blood donors in whom no risk of
HIV infection could be ascertained, who were nonreactive on the ELISA, and for whom all other tests for
HIV were negative, revealed that 20% to 40% might have an indeterminate Western
Blot... (Proffitt 1993, page 209)
Amazingly, the authors of the above study do not
even mention that this extremely high "indeterminate" reading might
raise questions about the specificity of the Western blot, which is currently
relied on heavily to diagnose AIDS.
* see Mark Craddock's
comments on Piatak et al infectious virus and QC-PCR numbers.
* If not treated, most people with HIV infection
show signs of AIDS within 5-10 years6,7.
HIV infection is identified in blood by detecting antibodies, gene sequences
or viral isolation. These tests are as reliable as any used for detecting other
virus infections.
COMMENT: Studies of long-term survivors or non-progressors
show that the majority do not take AIDS drugs and that
many of them refuse viral load tests as well as other surrogate marker tests
and lead normal lives - some of them since 1984 when HIV was first targeted
as "the probable cause of AIDS". (Buchbinder
1994, Cao 1995, Garbuglia
1996, Harrer 1996, Hogervorst
1995, Hoover 1995, Montefiori 1996, Pantaleo 1995, Root-Bernstein 1995).
Is the "HIV Test" Valid? The test kit manufacturer's
own literature admits:
"ELISA testing alone cannot be used to diagnose AIDS, even if
the recommended investigation of reactive specimens suggests a high probability
that the antibody to HIV 1 is present"
- Abbott Laboratories, 1994, 66-2333/R4.
The insert for one of the kits for administering the Western Blot warns:
"Do not use this kit as the sole basis of diagnosis of HIV-1 infection"
- Epitope/Organon Teknika
Corporation, PN201-3039 Revision # 6.
"Sensitivity and Specificity: At present there is no recognized standard
for establishing the presence and absence of HIV-1 antibody in human blood.
Therefore, sensitivity was computed based on the clinical diagnosis of AIDS
and specificity based on random donors. The ABBOT studies show that: Sensitivity
based on an assumed 100% prevalence of HIV-1 antibody in AIDS patients
is estimated to be 100% (144 patients tested). Specificity based on an assumed
zero prevalence of HIV-1 antibody in random donors is estimated to be 99.9%
(4777 random donors tested)."
- Abbott Laboratories HIVABtm
HIV-1 EIA
Assumed because it is not possible
to isolate HIV from fresh patient plasma. Thus it has never been
proven that any "HIV" antibody positive patient has an active "HIV"
infection.
The insert that comes with a popular kit to run viral load warns:
"The Amplicor HIV-1 Monitor test is
not intended to be used as a screening test for HIV or as a diagnostic test
to confirm the presence of HIV infection"
- Roche Diagnostic Systems, 06/96, 13-08-83088-001.
HIV test results vary depending on where you live.
In the
U.S., Canada, most of Europe but not in England or Wales, the Western Blot test
confirms ELISA tests. Dr. Valendar Turner explains
how HIV antibody test results can be interpreted very differently by nine different
international standards. For instance it is possible for any person to be any
of the following on a single test result: positive in Australia, but not in France; positive in France, but not in Australia (for different reasons);
positive in Africa and not positive everywhere
else in the world. A Martian might be forgiven for wondering whether wine tasting
was less subjective (Turner, 1995).
|
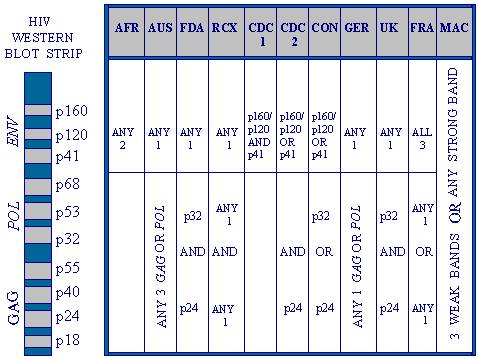
Criteria varying worldwide
for a positive HIV test result on Western Blot.
AFR = Africa; AUS = Australia; FDA = US Food and Drug
Administration; RCX = US Red Cross; CDC = US Center for Disease Control;
CON = US Consortium for Retrovirus
Serology Standardization; GER = Germany; UK = United Kingdom; FRA = France; MACS = US Multicenter AIDS Cohort Study 1983-1992.
Courtesy Dr. V. Turner
As for the recent thrust to test pregnant women
regardless of risk, the American Foundation for AIDS Research (AmFAR), among others, makes it clear that this idea will lead
to disaster:
Take, for example,
self selected and previously tested blood donors from the general population.
Here the prevalence of infection might be 1 case per 100,000 people … If the
test has a specificity of 99.9%, it means 0.1% -- about 100 of the test results
will be false positives. …
This means that a positive test result in this population has only a 1% chance
of being a true positive! (AmFAR, 1999)
|
